PRODUCTS
A Leading Molecular Diagnostics Company with Unrivaled Competitiveness


- Genetic Disorder Molecular Diagnostic Assay Kits
BioSewoom introduces its products developed through continuous research and innovation.
IVD Product License No: 12-1805
(Korea MFDS)
(Korea MFDS)
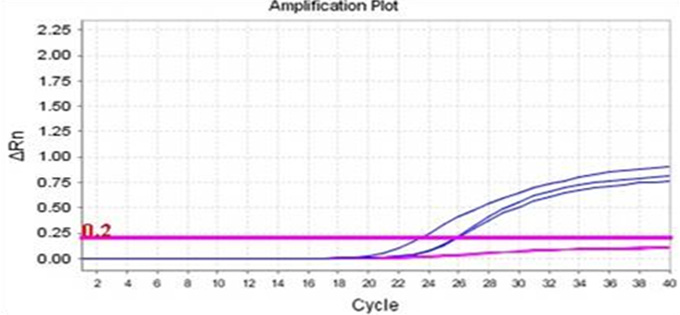
Real-Q Factor II(prothrombin)
G20210A Kit
G20210A Kit

IVD Product License No: 12-1806
(Korea MFDS)
(Korea MFDS)
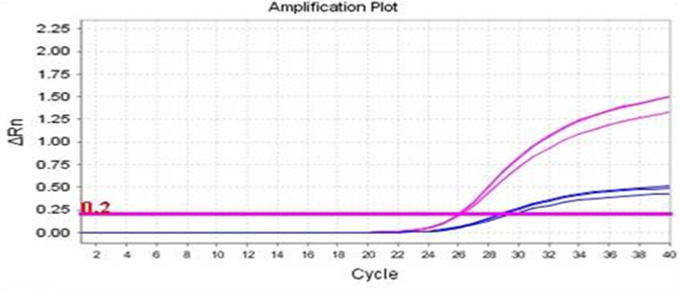
Real-Q Factor V Leiden(G1691A) Kit

IVD Product License No: 12-1807
(Korea MFDS)
(Korea MFDS)
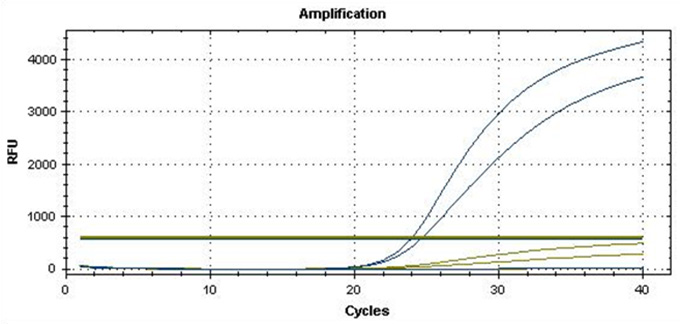
Real-Q MTHFR Kit
IVD Product License No: 17-955
(Korea MFDS)
(Korea MFDS)
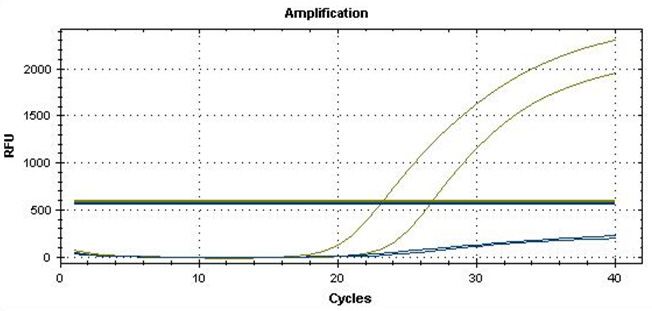
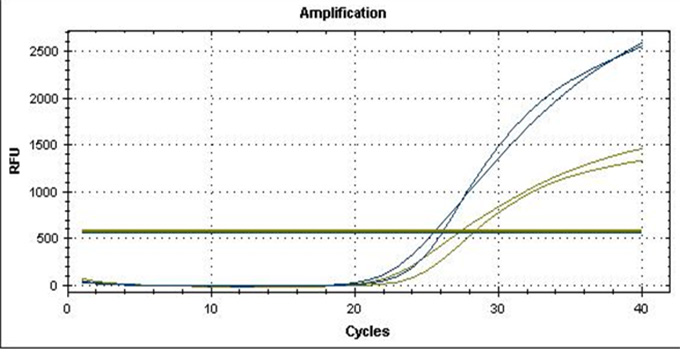
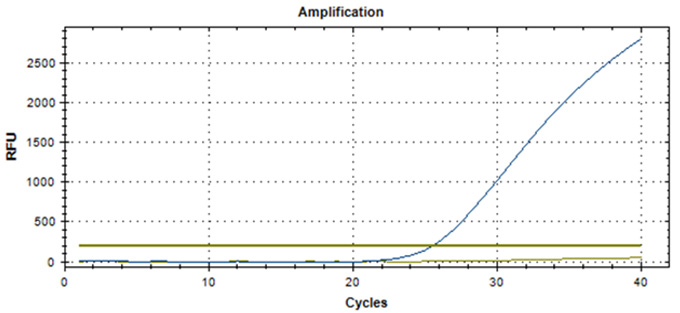
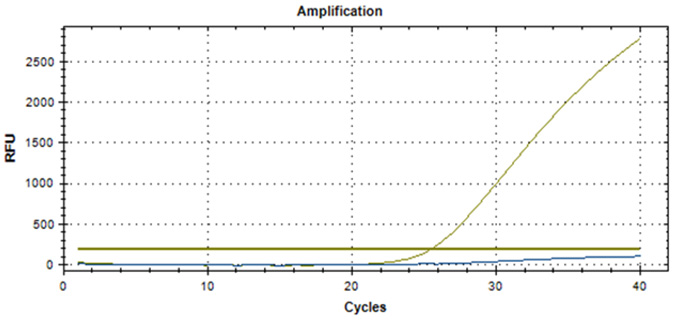
Real-Q RNF213 R4810K Kit





 ENG
ENG KOR
KOR