PRODUCTS
A Leading Molecular Diagnostics Company with Unrivaled Competitiveness


- HIV·HBV·HCV·HTLV Genotyping Assay Kits
BioSewoom introduces its products developed through continuous research and innovation.
IVD Product License No: 12-1743
(Korea MFDS)
(Korea MFDS)
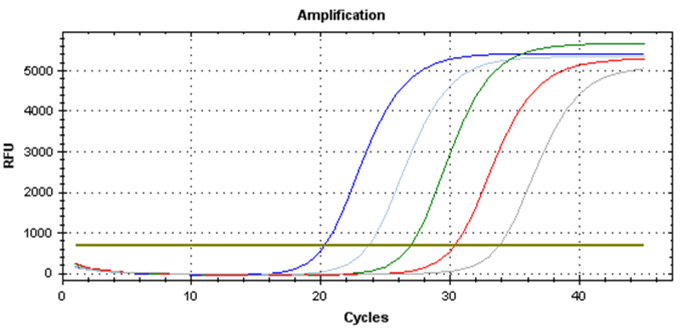
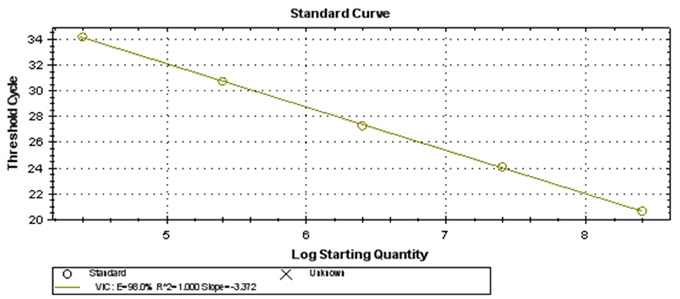
Real-Q HBV Quantification Kit


 ENG
ENG KOR
KOR