PRODUCTS
A Leading Molecular Diagnostics Company with Unrivaled Competitiveness


- Oncology-Related Genetic Assay Kits
BioSewoom introduces its products developed through continuous research and innovation.
IVD Product License No: 12-1736
(Korea MFDS)
(Korea MFDS)
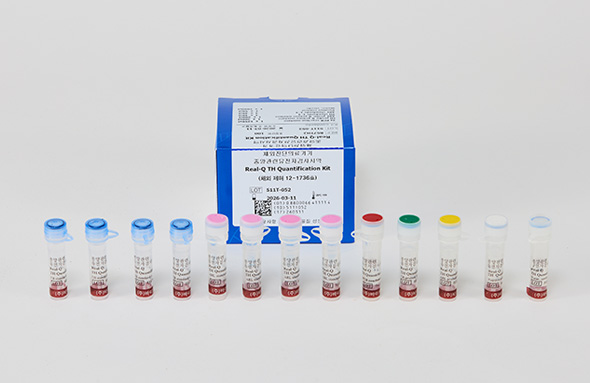
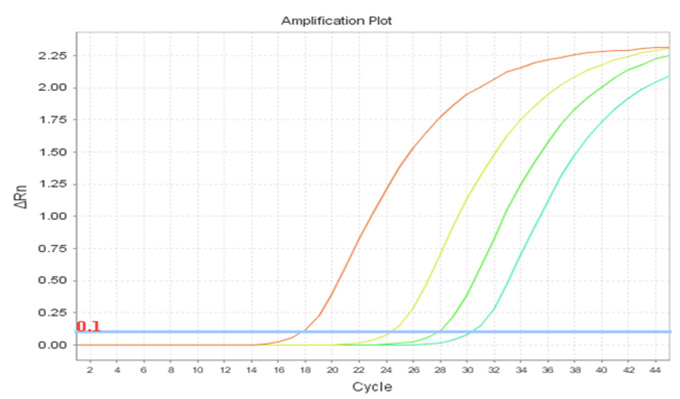
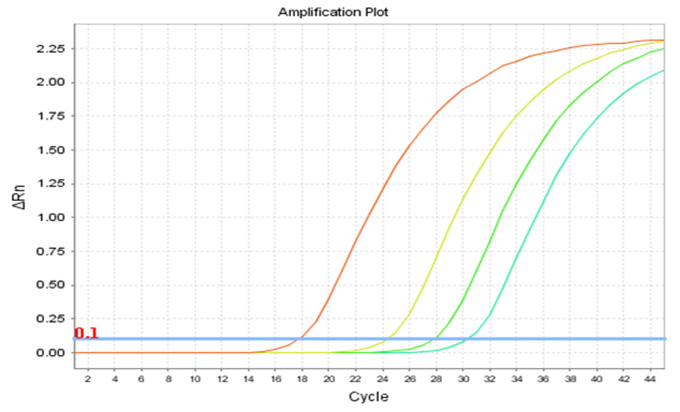
Real-Q TH Quantification Kit
IVD Product License No: 12-1757
(Korea MFDS)
(Korea MFDS)
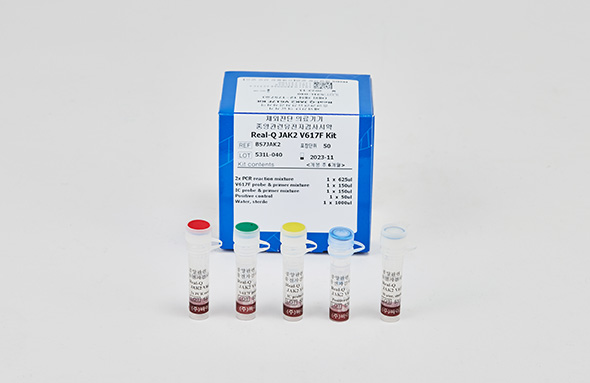
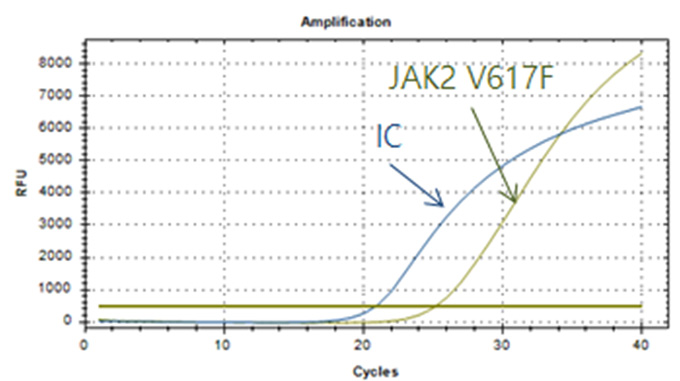
Real-Q JAK2 V617F Kit
IVD Product License No: 12-1758
(Korea MFDS)
(Korea MFDS)
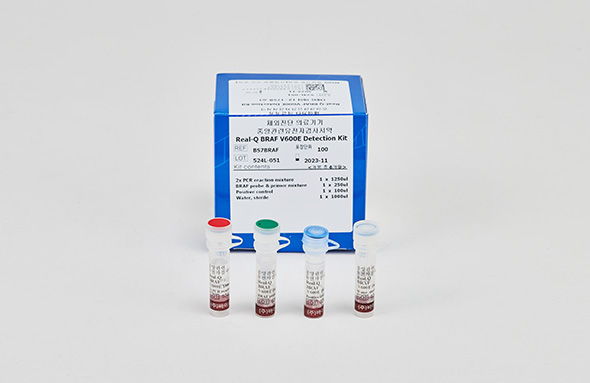
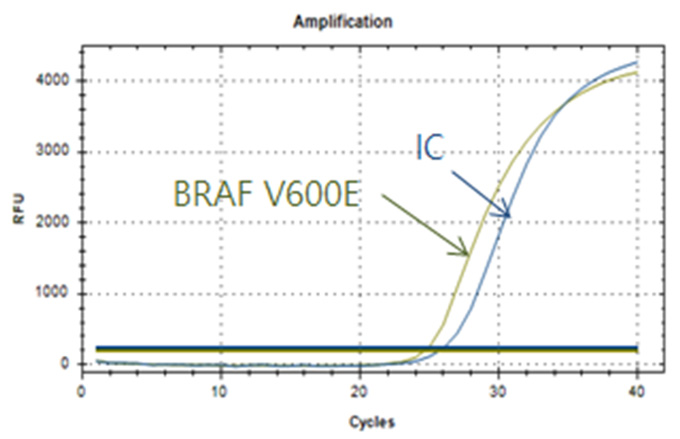
Real-Q BRAF V600E Detection Kit

IVD Product License No: 12-1761
(Korea MFDS)
(Korea MFDS)
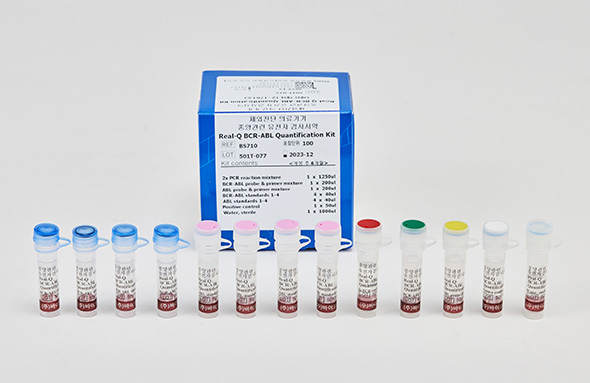
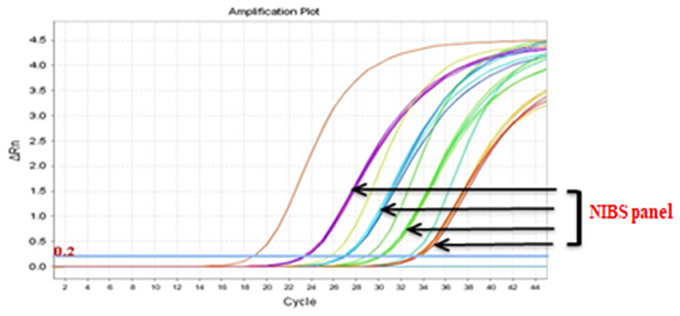
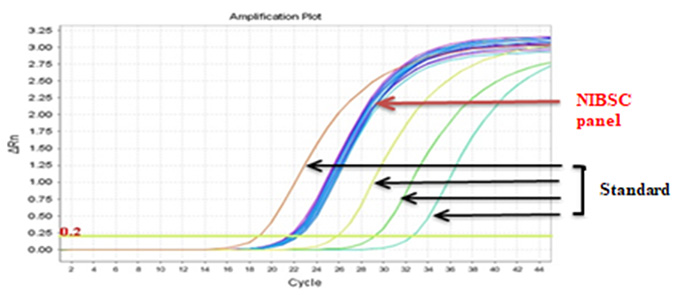
Real-Q BCR-ABL Quantification Kit
IVD Product License No: 12-1762
(Korea MFDS)
(Korea MFDS)
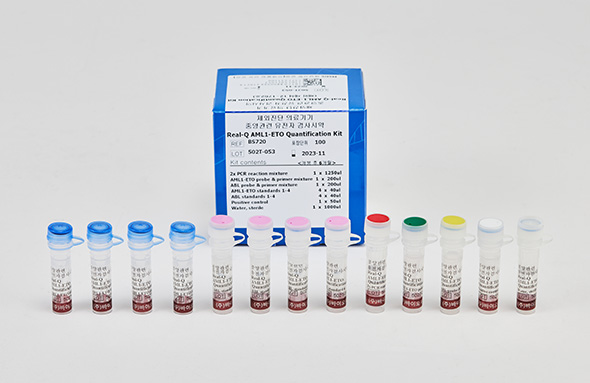
Real-Q AML1-ETO Quantification Kit
IVD Product License No: 12-1764
(Korea MFDS)
(Korea MFDS)
Real-Q NPM1-mutA Quantification Kit

IVD Product License No: 12-1770
(Korea MFDS)
(Korea MFDS)
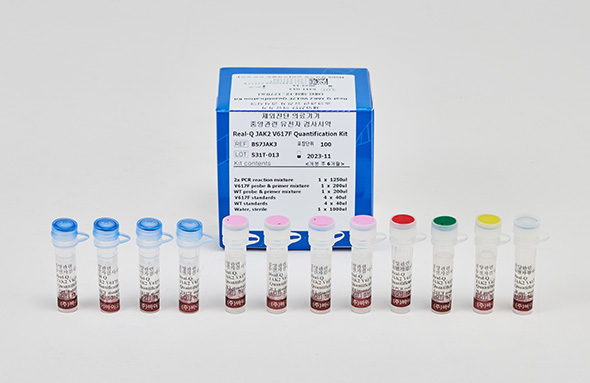
Real-Q JAK2 V617F Quantification Kit
IVD Product License No: 12-1785
(Korea MFDS)
(Korea MFDS)
Real-Q PML-RARa Quantification Kit
IVD Product License No: 15-1180
(Korea MFDS)
(Korea MFDS)
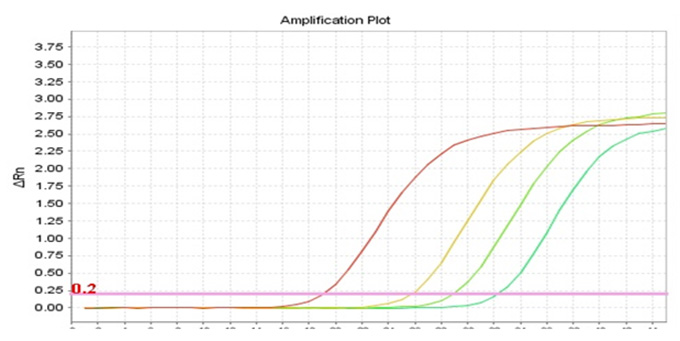
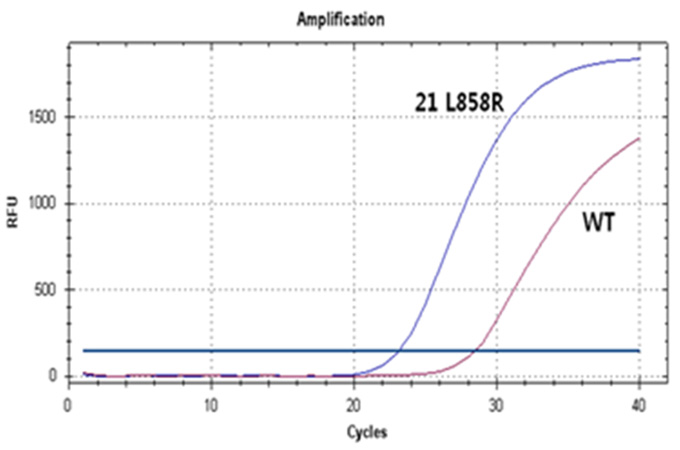
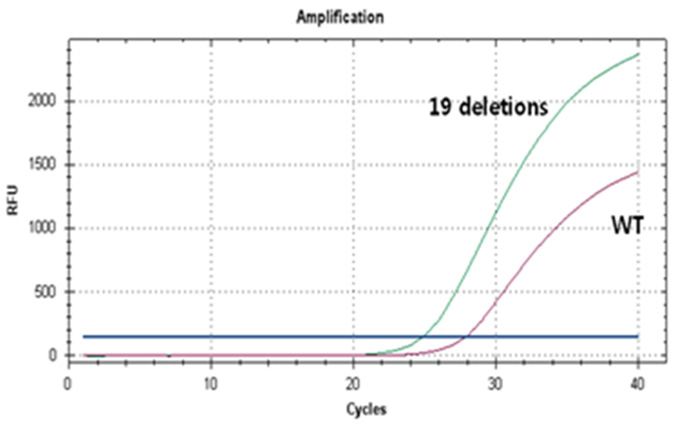
Real-Q EGFR Mutation Detection Kit
IVD Product License No: 15-1252
(Korea MFDS)
(Korea MFDS)
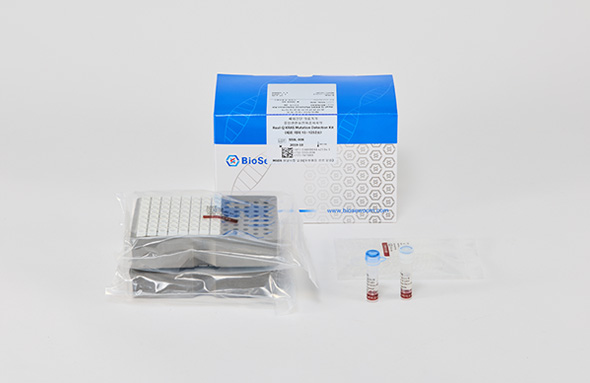
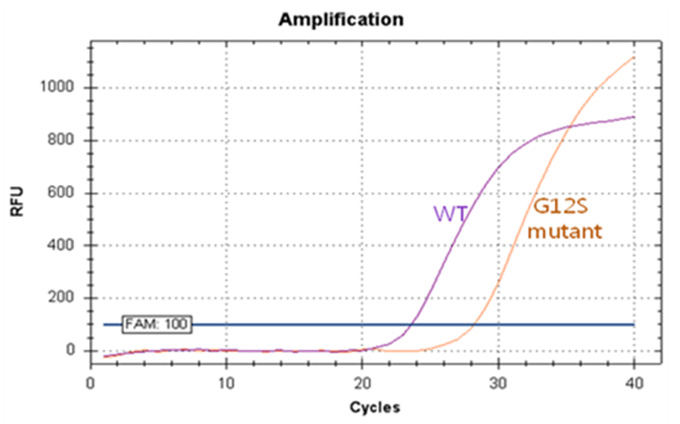
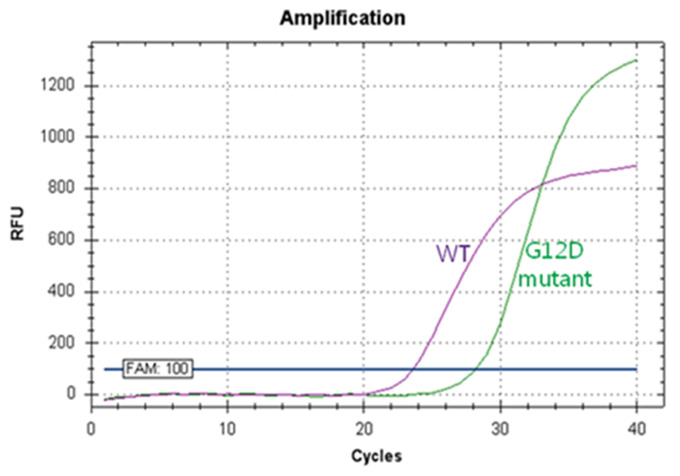
Real-Q KRAS Mutation Detection Kit





 ENG
ENG KOR
KOR