PRODUCTS
A Leading Molecular Diagnostics Company with Unrivaled Competitiveness


- HLA Typing Kits for Disease Diagnosis
BioSewoom introduces its products developed through continuous research and innovation.
IVD Product License No: 13-396
(Korea MFDS)
(Korea MFDS)
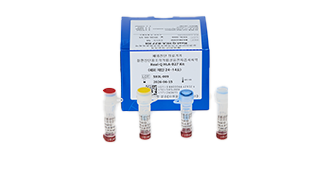
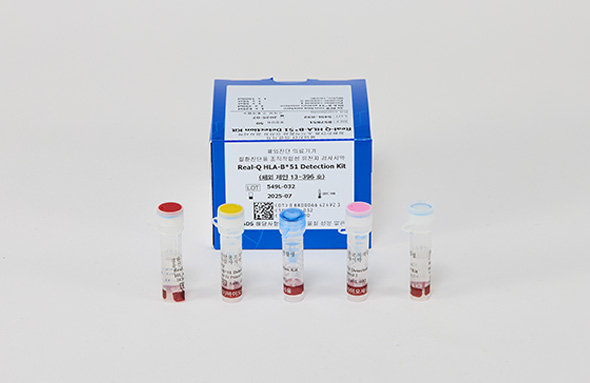
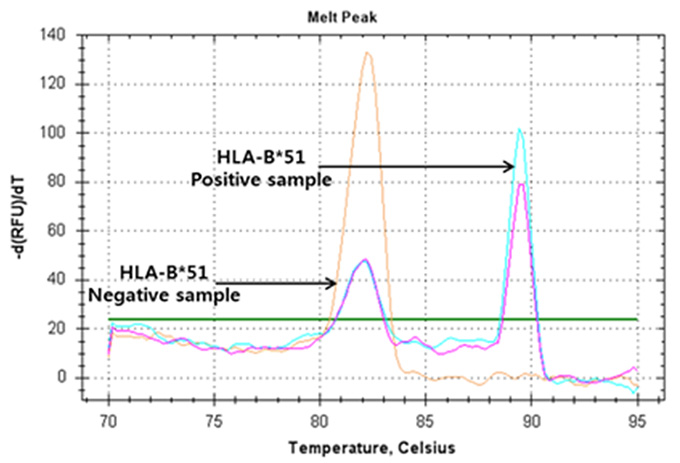
Real-Q HLA-B*51 Detection Kit

IVD Product License No: 13-397
(Korea MFDS)
(Korea MFDS)
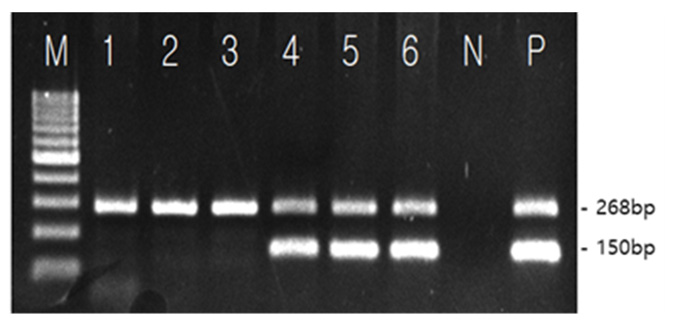
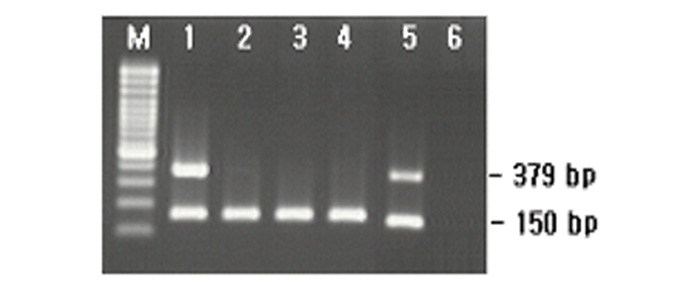
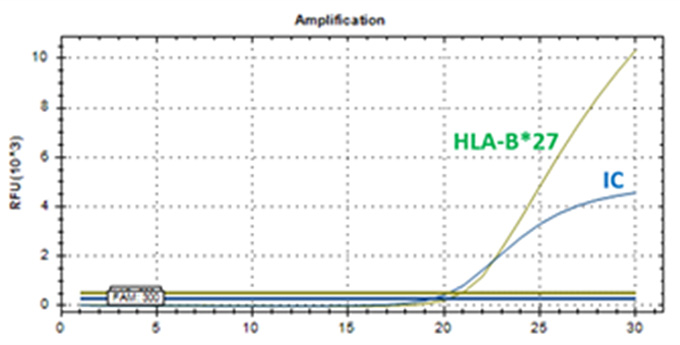
HLA-B*27 PCR Kit

IVD Product License No: 13-398
(Korea MFDS)
(Korea MFDS)
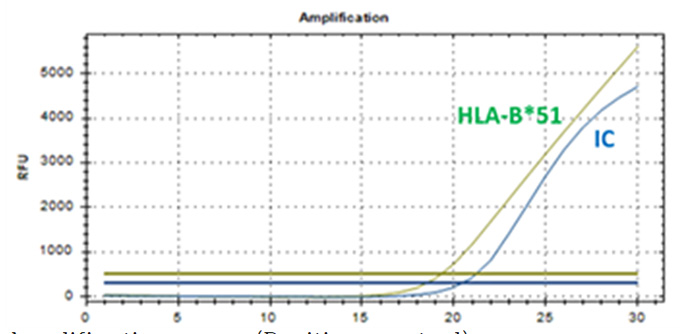
HLA-B*51 PCR Kit

IVD Product License No: 24-13
(Korea MFDS)
(Korea MFDS)
Real-Q HLA-B*51 Kit

IVD Product License No: 24-14
(Korea MFDS)
(Korea MFDS)
Real-Q HLA-B*27 Kit




 ENG
ENG KOR
KOR